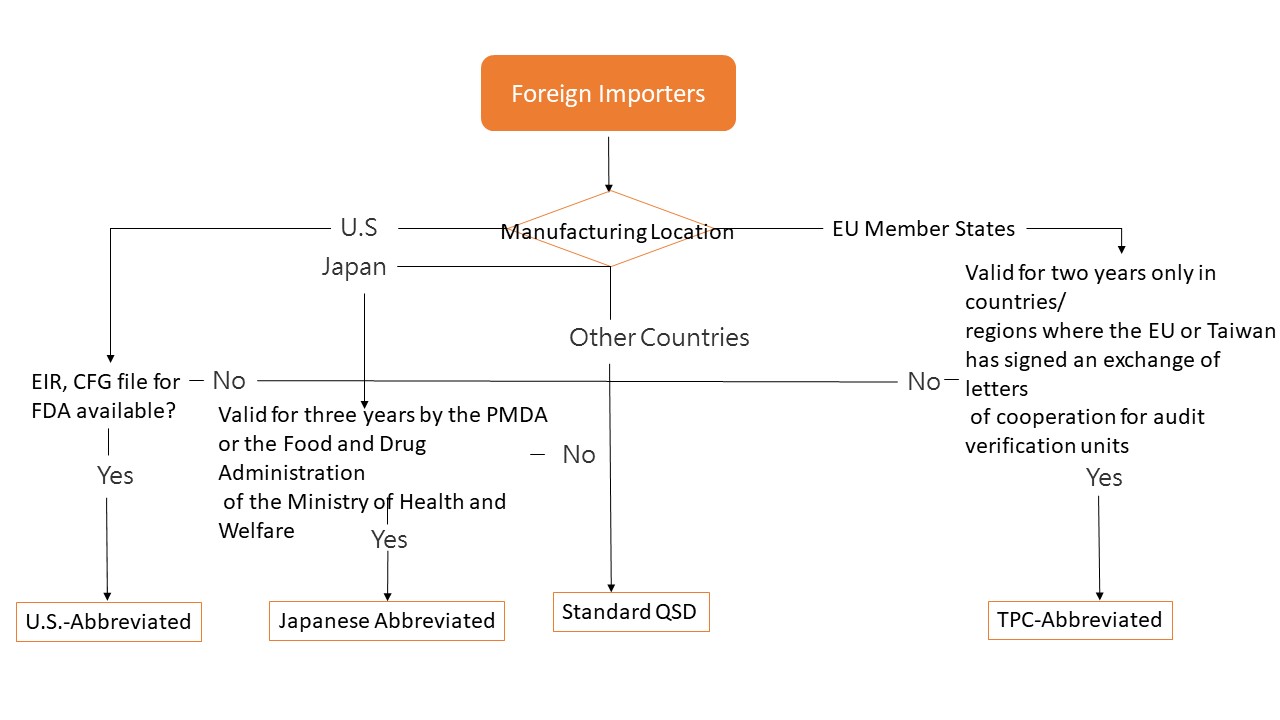
Medical devices imported into Taiwan must comply with the quality management system that harmonized with ISO13485, and submit the Quality System Documentation toTaiwan FDA.
According to the country of origin, the Application Pathway can be classified as:
●US-Abbreviated
●TCP-Abbreviated
●Japanese-Abbreviated
●Standard QSD
According to the country of origin, the Application Pathway can be classified as:
●US-Abbreviated
●TCP-Abbreviated
●Japanese-Abbreviated
●Standard QSD
Although abbreviated pathway seems more convenient, the requirements of the documentation is still varied . Also, the communication with the original manufacturer takes time. License Biomedical CO.,LTD can assist you in preparing the relevant documentation to obtain QSD approval with the highest efficiency.
Good Distribution Practice (GDP), ordered by MINISTRY OF HEALTH AND WELFARE is hereby given a 12 articles "Regulations for the Inspection of the Medical Device Good Distribution and Distribution Licenses" on 29 January 2021. Starting from 1 May 2021, the quality management system extends from production to the transportation and also sales, to ensure the product quality and the packaging integrity during the storage, transportation and sales process. It is a serious issue of medical devise. Considering certification of GDP for your products is a must.


