All medical devices that intend to apply for marketing registration need to undergo clinical trials to prove their safety and efficacy under normal use in order to obtain market approval for medical devices. High-risk or innovative medical devices will emphasize the requirement of clinical trial.
Our CRO consultant team complies with international medical regulations and ICH guidelines to perform high-standard clinical trial for you to ensure that your clinical reports comply with clinical plans and regulatory requirement.
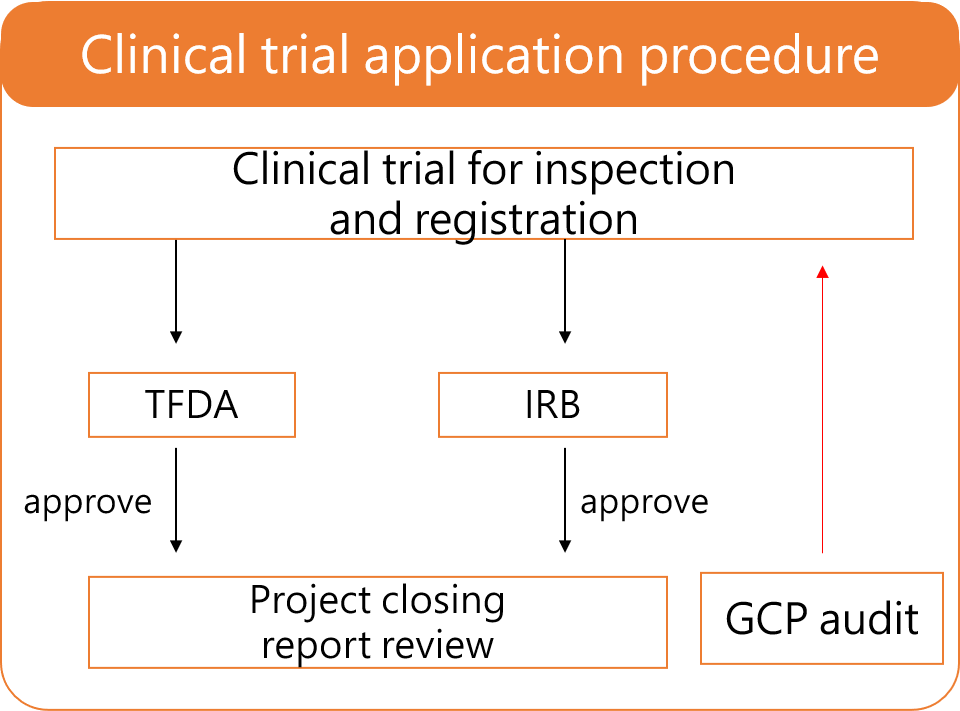
Our CRO consultant team complies with international medical regulations and ICH guidelines to perform high-standard clinical trial for you to ensure that your clinical reports comply with clinical plans and regulatory requirement.

License Biomaterial CO.,LTD. provides you the following services:
●Clinical Research organization (CRO) matching and clinical trial planning
●Submissions for IRB
●Project management, monitoring and test progress report
●Submissions for the health authority
●Clinical evaluation report writing
●Clinical Research organization (CRO) matching and clinical trial planning
●Submissions for IRB
●Project management, monitoring and test progress report
●Submissions for the health authority
●Clinical evaluation report writing


